On 15 November 2023, the team of Prof. Xie Huiqi from the Laboratory of Stem Cell and Tissue Engineering, State Key Laboratory of Biotherapy at West China Hospital, Sichuan University published an original article titled “Promotion of uterine reconstruction by a tissue engineered uterus with biomimetic structure and extracellular matrix microenvironment” in Science Advances (IF: 13.6). In this study, the team constructed a tissue engineered uterine repair scaffold with structure and extracellular matrix (ECM), and systematically investigated the role of tissue specific ECM in recruiting endogenous stem cells and inducing their directed differentiation, demonstrating the feasibility of this bionic scaffold for the repair of severe intrauterine adhesions (IUA).

IUA is the major cause of secondary infertility for women, but traditional treatments are ineffective. The recurrence rate of severe IUA is up to 60%, and more effective methods of preventing and treating IUA remain to be developed. Severe IUA often results in myometrial damage, leading to local ischemia and hypoxia, severely impeding uterine repair. Therefore, in the treatment of severe IUA, it is crucial to give appropriate mechanical support, establish an anti-adhesion barrier, and achieve simultaneous regeneration of the endometrium and myometrium. However, it is difficult for a single scaffold to meet the multiple demands of severe uterine injury repair. Inspired by the structure of the uterus, this study developed a bilayer scaffold (ECM-SPS) with bionic heterogeneous properties and extracellular matrix microenvironment to meet the multiple repair needs of the uterus. The bionic scaffold has two distinct regions that resemble the anatomy of the uterus, with a dense and smooth surface of the bionic endometrium (SIS), which serves as a physical barrier against adhesions. The bionic myometrium (PU/SIS) has similar mechanical properties to the myometrium and can provide suitable mechanical support (Fig. 1). It has been demonstrated that this scaffold can maintain normal uterine morphology and prevent the occurrence of stenosis or adhesions in the repair of total uterine defects.
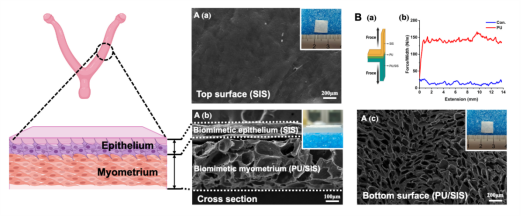
Fig. 1
Loss of endometrial stem cells and substantial alterations in the tissue microenvironment are considered to be the main causes of uterine pathological regeneration. The function of endometrial stem cells is largely limited by the microenvironment at the repair site, and the recruitment of endogenous stem cells and the construction of a favorable repair microenvironment are expected to achieve functional uterine regeneration. As an important component of the tissue microenvironment, ECM can regulate cell phenotype and function in response to various physiological and pathological stimuli, especially inducing stem cell lineage differentiation. ECM are usually derived from animal tissues, however, their batches are highly varied and face more immune rejection and biosafety risks. This study used tissue-specific cells to produce ECM to mimic the natural tissue ECM microenvironment and induce functional tissue regeneration. It was found that tissue-specific cell-derived ECM had ECM characteristics similar to those of natural tissues and could mimic the tissue ECM microenvironment to induce stem cell directed differentiation. In addition, uterine epithelial-derived ECM could recruit endogenous stem cells to participate in endometrial repair (Fig. 2 B), and smooth muscle cell-derived ECM showed more promotion of neovascularization (Fig. 2 C).
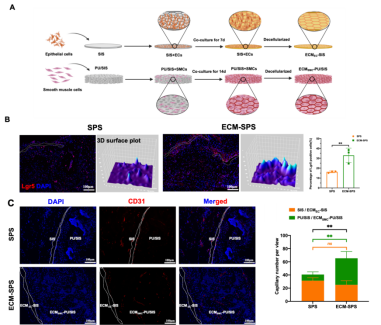
Fig.2
In the subtotal semicircular uterine defect model, the bionic scaffold demonstrated rapid epithelialization and promoted regeneration of endometrial glands and myometrium (Figure 3 A-B). In addition, the bionic scaffold had no effect on ovarian function, and the estrous cycle and follicle number did not differ from those of normal rats. The reconstructed uterus with the bionic scaffold could maintain embryonic gestation and live birth of fetuses with a higher conception rate (Figure 3 C).
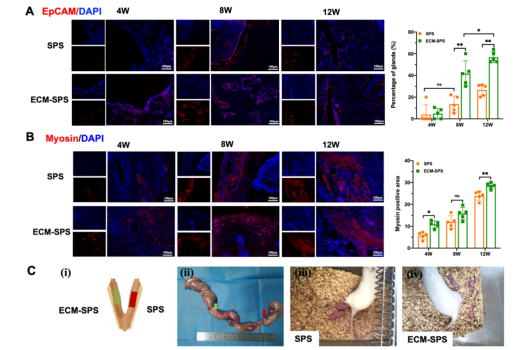
Fig.3
This study suggests that ECM-SPS has a great potential for the treatment and prevention of severe IUA. The structure and microenvironment biomimetic ECM material construction strategy proposed in this study provides better options for organ regeneration of complex tissue structures.
Link:
https://www.science.org/doi/10.1126/sciadv.adi6488?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed