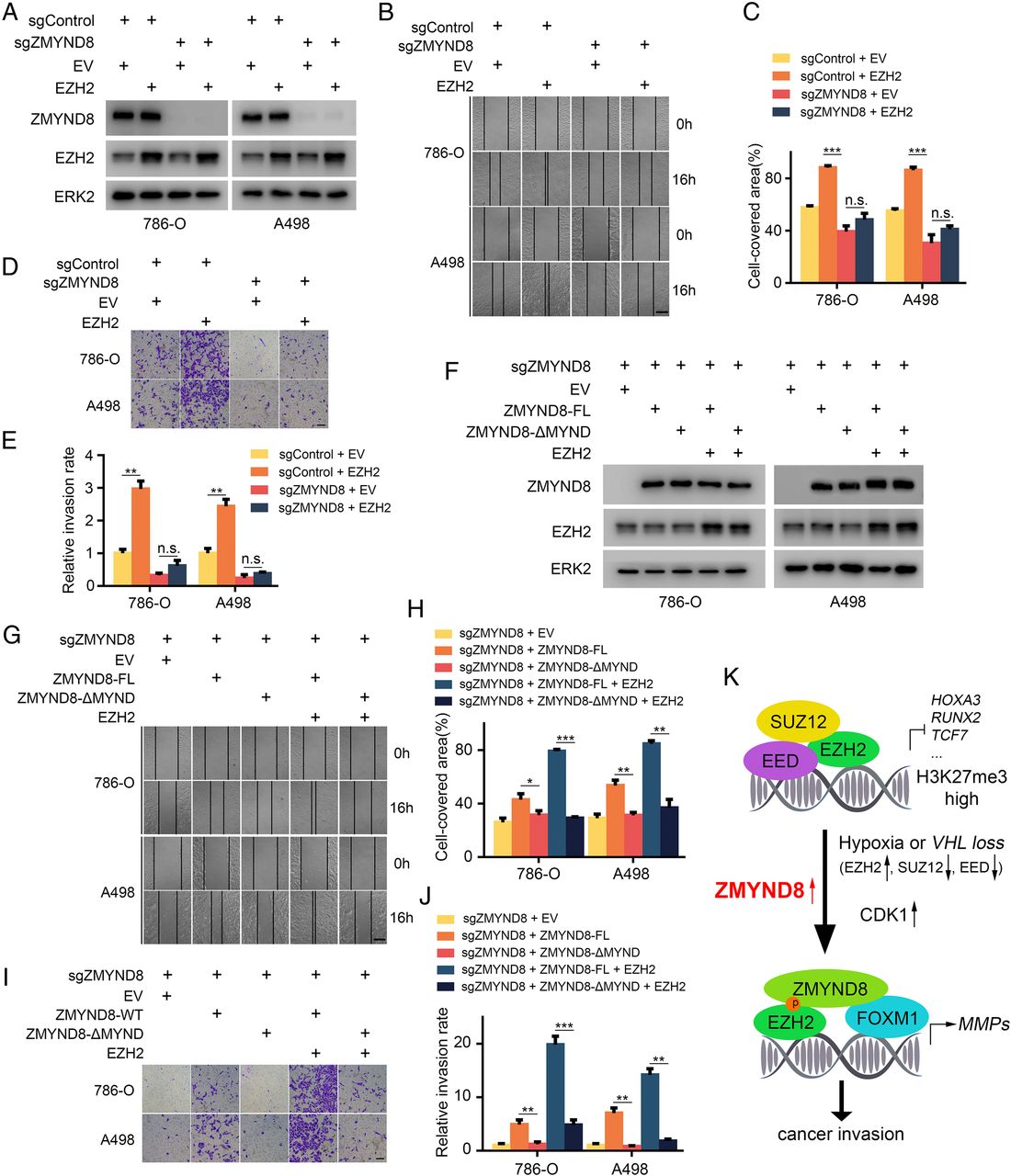
Recently, Prof. Wei Qiang’s team from the Urology Department of West China Hospital collaborated with Prof. Huang Haojie from Mayo Clinic and published a research paper named “ZMYND8 Preferentially Binds Phosphorylated EZH2 to Promote a PRC2-dependent to-independent Function Switch in Hypoxia-inducible Factor-activated Cancer” online in PNAS (IF: 9.4), an internationally-renowned academic journal, revealing the new functions of ZMYND8.
As a histone modification ‘reader’, ZMYND8 can recognize histone methylation, acetylation and even some histones with specific mutations, and involve in cell transcription regulation. This study found that ZMYND8 is overexpressed in human clear cell renal cell carcinoma and preferentially binds to T487 phosphorylated EZH2. It also showed that in hypoxia-exposed or von Hippel-Lindau-deficient cancer cells, the combination of EZH2 and ZMYND8 is very important to maintain the phosphorylation level of EZH2, inhibiting the formation of PRC2 complex, and enhancing the interaction between EZH2 and FOXM1. ZMYND8 not only inhibits the transcriptional inhibition function of EZH2 under hypoxia or von Hippel-Lindau (VHL) deletion conditions, but also involves in the transcriptional activation of ZMYND8-EZH2-FOXM1 complex, mediating cancer cell invasion and metastasis. The results revealed a new function of ZMYND8: recognizing the phosphorylation “code” of non-histones, so it could play an important role in the functional transformation of EZH2 from transcriptional inhibition to transcriptional activation.
This project is an in-depth study on the mechanism of invasion and metastasis of renal cell carcinoma. The research results can give us a better insight into the mechanism and key molecules of invasion and metastasis in renal cell carcinoma, and may eventually provide a new direction for the treatment of renal cell carcinoma and molecular targeted drug development.